Process Control for Advanced Pharmaceutical Manufacturing (CESPE Academy)
Description
Course takes place on 23rd and 24th of April 2026 at the Faculty of Pharmaceutical Sciences (Ottergemsesteenweg 460, B-9000, Ghent).
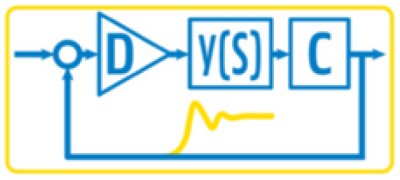
Pharmaceutical manufacturing is undergoing a paradigm shift, evolving from Quality by Design (QbD), where quality is built into the process during development to Quality by Control (QbC), where advanced control strategies and real-time monitoring actively ensure product quality during production. The training addresses the question of how we can keep pharmaceutical processes in optimal manufacturing conditions while ensuring versatility, resilience and quality of end product.
This intensive 1.5-day course, given by experts from Dynamical Systems and Control (DySC) lab, provides a practical and scientifically grounded framework for modern process control and optimization in pharmaceutical manufacturing. Participants will gain hands-on experience using a virtual plant simulation environment, where they will design, test, and evaluate different control strategies in a safe, risk-free setting.
This course is divided into two separate modules:
- Module 1: Fundamentals & Classical Control (full day):
Introduction to Process Control in Pharma
Identification at operator level (e.g. first order plus dead time approximation model)
Hands-on modelling session (identify FOPDT model of unit operations)
PID Control Fundamentals
Control structures and PID design
Hands-On Simulation Session (Implementing and tuning PID controllers in the virtual plant)
- Module 2: Advanced Control (half day)
Limitations of PID & Need for Advanced Control
Introduction to Model Predictive Control (MPC)
Hands-On: Implementing MPC (setting up MPC for the same virtual plant)
Moving towards real-world implementation and QbC readiness
Target Audience
- Researchers and operators with no prior experience in process modeling or control.
- Process engineers, quality professionals, control engineers, and R&D scientists working in pharmaceutical manufacturing.
- Professionals seeking a refresher on process identification and control principles.
Programme
Remarks
Additional information
- One week prior to the course, the registered participants will receive further information (directions, parking availabilities, course material).
- Upon completion of the training, a certificate of attendance will be made available on the platform.
- Additionally, the participant will receive an evaluation form to provide feedback on this training.
- By registering for this course, the participants consent to the sharing of their name, email, job position, and the level of their familiarity with the topic of the course with the course instructor/provider for the purposes of tailoring the course's contents and sharing educational course-related information.
- Ghent University is recognized as a training provider. With training vouchers from the Flemish Government, your company can save up to 30% on the participation fee for our courses. You can apply for the KMO Portfolio subsidy from VLAIO online at www.kmo-portefeuille.be for financial support to entrepreneurs for purchasing training and consultancy services at CESPE Academy no later than 14 calendar days after the start of the course. For more information, please see https://www.ugent.be/nl/opleidingen/levenslang-leren/kmo. Use the following authorisation-ID when requesting the subsidy: DV.O103194.
- For further information or any questions related to the course, please contact Mark.Gontsarik@UGent.be
Annullation/Cancellation
Registration is possible up to one week before the day of the respective session.
Registrations can only be cancelled by email. Cancellations earlier than 2 weeks before the course start will be fully refunded. In case the cancellation occurs later than two weeks before, but earlier than one week before the start of the course, half of the registration fee will still be due. In case of cancellation less than one week before the start of the course, the full registration fee will still be due. For UGent PhD students, the price of the registration fees for the Academic (UGent postdoc or professor) category will be considered when calculating the cancellations fees.
The organization reserves the right to reschedule or cancel the training in case of insufficient number of participants.
Pricing (for full 1.5-day course)
- Industry (non CESPE member): 1080.- EUR
- Industry (CESPE member): 760.- EUR
- Academic (non-UGent): 540.- EUR
- Academic (UGent postdoc or professor): 430.- EUR
- Academic (UGent PhD student): 0.- EUR (free of charge). Add the course to your card and specify ‘Academic (UGent PhD student)’. The discount will be applied during the checkout.
Note: the two modules can be taken separately.
Register here
Process Control for Advanced Pharmaceutical Manufacturing - Full Course
This course will provide interactive learning and real-world application, so you can directly apply what you’re learning to your own context
MODULE 1: Fundamentals & Classical Control (full day)
- Introduction to Process Control in Pharma
- Identification at operator level (e.g. first order plus dead time approximation model)
- Hands-on modelling session (identify FOPDT model of unit operations)
- PID Control Fundamentals
- Control structures and PID design
- Hands-On Simulation Session (Implementing and tuning PID controllers in the virtual plant)
MODULE 2: Advanced Control (half day)
- Limitations of PID & Need for Advanced Control
- Introduction to Model Predictive Control (MPC)
- Hands-On: Implementing MPC (setting up MPC for the same virtual plant)
- Moving towards real-world implementation and QbC readiness
Inschrijven
1st Module: Fundamentals & Classical Control (full day)
Only MODULE 1: Fundamentals & Classical Control (full day)